Most atoms do not have eight electrons in their valence electron shell. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations. Most metals become cations when they make ionic compounds.
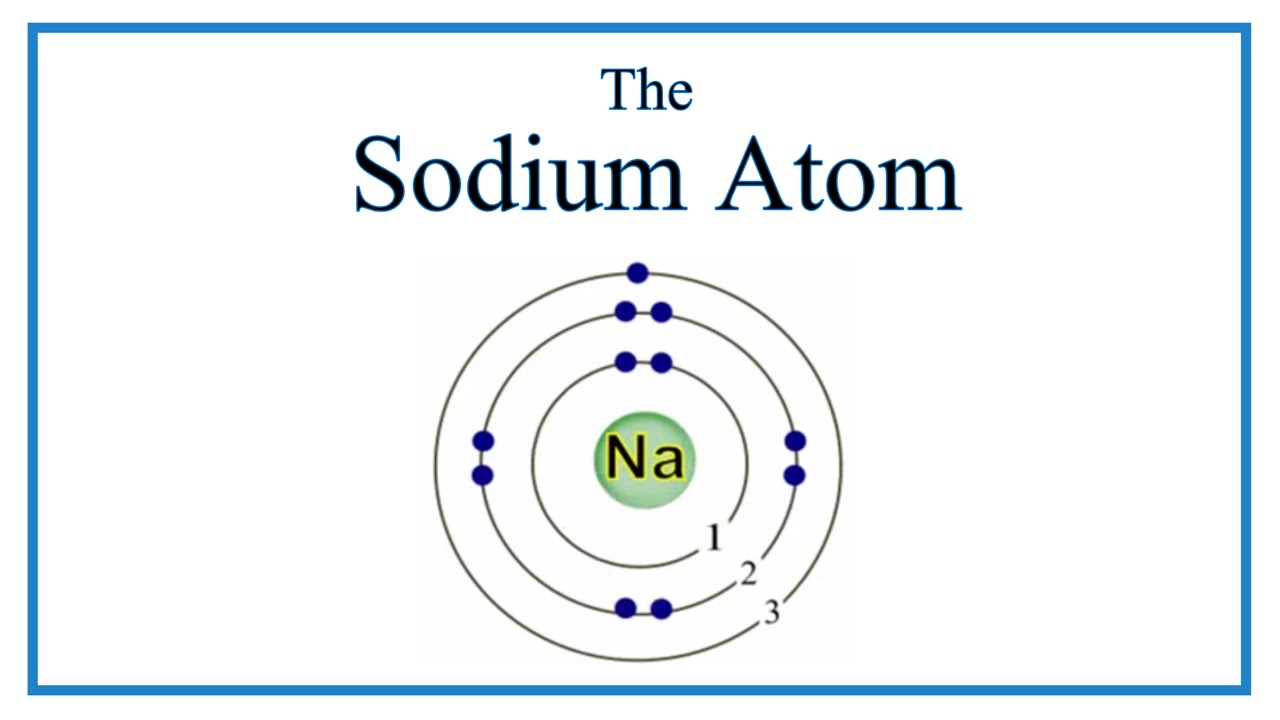
In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. 1s2, 2s2, 2p6, 3s1 is the electron configuration of Sodium, Na. The amount of electrons that Na has has to be reduced by one, as Na+1 lacks one electron from Na. So Na+1: 1s2, 2s2, 2p6. Sodium has one valence electron. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. The third shell, which is the outermost and the valence shell, has only one electron. Valence electrons influence chemical reactivity. Sodium donates the electron to the gap, even though the electron doesn't bond to the ammonia. In this manner, sodium (or another active metal, such as Na, K, Ba, or Ca) can donate an electron and liquid ammomia doesn't have to pick it up. This leaves an uncoupled electron in solution, the solvated electron. Atomic number of sodium is 11. Atomic number signifies, number of electrons or protons present in the system. Since, atomic number of sodium is 11, the electrons present in.
Cations
A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron.
Na Electrons
[ce{Na rightarrow Na^{+} + e^{-}}]
The cation produced in this way, Na+, is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it. The octet rule has been satisfied. Figure (PageIndex{1}) is a graphical depiction of this process.
Na Electrons Number
Anions
Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Negatively charged ions are called anions. Most nonmetals become anions when they make ionic compounds.
A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, this electron comes from the sodium atom.)
[ce{e^{-} +Cl -> Cl^{-}}]
In this case, the ion has the same outermost shell as the original atom, but now that shell has eight electrons in it. Once again, the octet rule has been satisfied. The resulting anion, Cl−, is called the chloride ion; note the slight change in the suffix (-ide instead of -ine) to create the name of this anion. Figure (PageIndex{2}) is a graphical depiction of this process.
The names for positive and negative ions are pronounced CAT-eye-ons and ANN-eye-ons, respectively.
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Figure (PageIndex{3}) shows how the charge on many ions can be predicted by the location of an element on the periodic table. Note the convention of first writing the number and then the sign on a ion with multiple charges. The barium cation is written Ba2+, not Ba+2.
Na Electrons Ion
Contributions & Attributions
Na Electrons In Outer Shell
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
